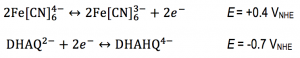A group under the Engineering department at Aarhus University is working with solid state and flow batteries. This article is a description of the groups focus and accomplishments.
Introduction and focus
Our group aims at developing technology for future low cost storage of energy. The technology of choice is stationary batteries for storage of renewable electricity that has the potential to reach
capital costs below 100 € kWh-1 and a lifetime that gives storage cost below 5 ¢ kWh-1 cycle-1
(incl. assembly, installation and maintenance).
 The main motivation for developing cheaper electrical energy storage technologies is the expectation of a complete shift away from fossil-based electricity generation in the near future, especially in Europe, which is both politically and economically motivated. Storage cost below 5 ¢ kWh-1 cycle-1 is a game changer within electrical energy storage. It will make it economically feasible to use stationary batteries as intermediate storage of renewable energy from wind turbines or solar panels for its later use in, e.g., electric vehicles and buildings.
The main motivation for developing cheaper electrical energy storage technologies is the expectation of a complete shift away from fossil-based electricity generation in the near future, especially in Europe, which is both politically and economically motivated. Storage cost below 5 ¢ kWh-1 cycle-1 is a game changer within electrical energy storage. It will make it economically feasible to use stationary batteries as intermediate storage of renewable energy from wind turbines or solar panels for its later use in, e.g., electric vehicles and buildings.
The focus is obtaining the lowest costs possible by using:
- New low cost redox species for solid state and flow batteries
- Direct solar charging of flow batteries
In addition, the materials should be environmentally benign and safe to handle. In traditional battery technology, toxicity has not always been considered, and batteries generally have to be collected and destroyed responsibly after their lifetime. By careful choice of redox couples that can e.g. be derived from rhubarb (quinones), it may be possible to create a safer technology. We target to develop prototypes (kWh scale) of both Ni(OH)2/anthraquinone solid state batteries and all organic, water soluble, quinone based flow batteries.
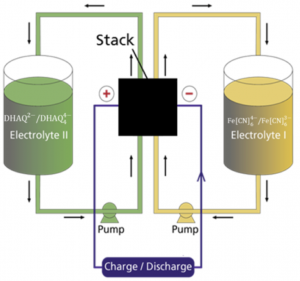
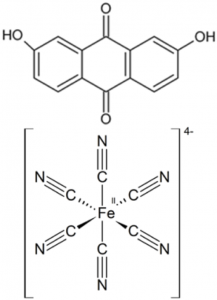
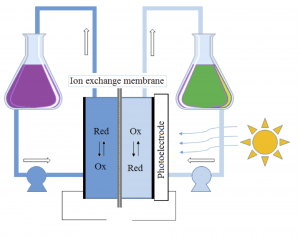
Figure 1: Schematic figure of the flow battery and the molecular structures of the redox species anthraflavic acid (top right) and ferrocyanide (bottom right)
A flow battery (Figure 1) basically works the same way as a fuel cell, but with the difference that the electricity is stored in liquids in two separate tanks, as opposed to hydrogen and oxygen in fuel cells, and the operating temperature is usually room temperature. Currently we are using the redox active species ferrocyanide () as a positive side and anthraflavic acid (2,6-dihydroxy anthraquinone or DHAQ) as a negative side, both in 1 M KOH aqueous supporting electrolyte. The specific redox reactions are:
and this results in a formal cell potential of 1.1 V.
Although ferrocyanide contains cyanide groups, it is a completely harmless and environmentally friendly chemical and it is even used as an additive for table salt. The components of the redox active species (both sides) and solvent consists only of abundant and environmentally friendly element (K, C, H, N, O and Fe). As seen from the redox reactions of ferrocyanide and anthraflavic acid both species are negatively charged therefore cation exchange membrane, typically Nafion, is used to effectively reduce the crossover of redox species in the flow battery. During charging/discharging K+ is exchanged through the membrane, while electrons are passed through an external circuit.
The drawback of this battery chemistry is a low solubility of potassium ferricyanide and anthraflavic acid in water, i.e., 0.4 M and 0.5 M in 1 M KOH, respectively. We are currently advancing the solubility of both species and it is our aim to improve the energy density from 11 to 25 WhL-1.
All organic flow battery
Despite the promising properties (chemical stability and electrochemical reversibility) of the redox active species, anthraflavic acid and ferrocyanide we do aim to develop all organic flow battery. To the best of our knowledge there is no stable organic redox compound known for the positive side. The key issues with the positive side are water solubility and chemical stability. For example, some of the positive organics we tested is water soluble TEMPO radical which degrades during charging/discharging. For this reason we target the development of large water soluble, mainly quinone based organic redox species. This is a work in a co-operation between our group (dr. Emil Drazevic), an organic synthesis group in Budapest, led by dr. Denes Konya, and an organic synthesis group at AU, led by Anders Thyboe Lindhardt. To achieve our goal of finding the best possible “not from the shelf” candidate, we follow two different methodologies. (i) The first approach is to modify existing hydroquinone structures in order to achieve better solubility and stability. In this case the hydroquinone moiety is substituted with hydrophilic groups to increase solubility. (ii) The second methodology concerns modifying and testing core structures where instead of the Oxygen (Quinone derivatives) the nitrogen is the containing core structures like viologen, NAD and FAD analogues. These structures have per se higher solubility.
PhD students
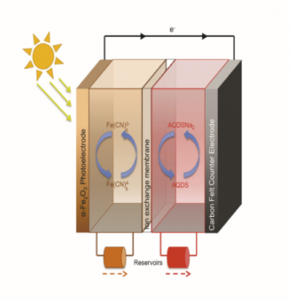
Figure 2. Solar charging of redox flow batteries. Positive side, ferrocyanide, negative side sodium anthraquinone disulfonic acid.
Our PhD students, Kristina Wedege and Amirreza Khataee are currently using similar redox couples for direct solar charging of redox flow batteries as a relatively new (forgotten) technology capable of simultaneously harvesting and storing energy from the sun for subsequent conversion into electricity (Figure 2). They are using photo-electrochemical principles to store the energy in two liquids. These liquids are pumped into a flow battery where the energy is converted into electricity. The technology works according to the same principle as fuel cells where hydrogen is produced photo-electrochemically from water (Figure 3). The technology for solar charging is both simple and inexpensive because the flow battery’s photo electrode is made of a glass substrate coated with a solar active metal oxide, e.g. iron oxide in hematite form – commonly known as rust. Our recent work (accepted for publication) has demonstrated that the principle of charging flow batteries directly with solar radiation works, however the efficiency is still low. The goal of Kristina’s and Amirreza’s project is to use low cost materials and optimize all process parameters. They should be also able to answer the research question: Is it possible to experimentally show high efficiencies (hc > 10 %) of solar charging of redox couples?
 Development of cheaper, more robust membranes is a key issue in flow battery development, as the cost of the membrane is high. Changing the focus to organic redox species may warrant a change away from Nafion to cheaper kinds of separators, for instance size exclusion or new types of ion conductive membranes. We are therefore working on development of new low cost membranes for flow batteries. The work is currently being performed by PhD student Mette Birch Kristensen and assistant professor Jacopo Catalano.
Development of cheaper, more robust membranes is a key issue in flow battery development, as the cost of the membrane is high. Changing the focus to organic redox species may warrant a change away from Nafion to cheaper kinds of separators, for instance size exclusion or new types of ion conductive membranes. We are therefore working on development of new low cost membranes for flow batteries. The work is currently being performed by PhD student Mette Birch Kristensen and assistant professor Jacopo Catalano.
Vanadium Redox Battery System
Finally, we possess a 5 kW/5kWh All Vanadium Redox Battery System in the lab, which was assembled mainly by Niels Vinther Voigt, postdoc and employer of spinout company VisBlue, and Luis Carlos Perez Matinez, postdoc.
People in the group
Anders Bentien, Associate Professor and group leader
Jacopo Catalano, Assistant Professor in Ion Exchange Membrane Engineering
Luis Carlos Perez Matinez, Postdoc, developing a large scale All Vanadium Flow Battery
Emil Drazevic, Postdoc, (Marie Curie Sklodowska Individual Fellowship) developing All Organic Redox Flow Batteries and solid state Ni(OH)2/anthraquinone batteries.
Kristina Wedege, PhD, Solar Charged Flow Batteries
Amirezza Khataee, PhD, Solar Charged Flow Batteries
Casper Clausen, PhD, solid state Ni(OH)2/anthraquinone batteries
Mette Birch Kristensen, PhD, development of ion-exchange membranes
Søren Duch-Hennings, MSc, solid state Ni(OH)2/anthraquinone batteries
Roar Larsen, BSc, optimization of flow cells for redox flow batteries