Using high-intensity X-rays to study the crystal structure of a common battery material while it charges and discharges, researchers have filled in new details about how mechanical strain hinders battery performance. These insights could help battery chemists tailor the composition of electrode materials to make better high-power batteries.
This article is co-authored by Dorthe Bomholdt Ravnsbæk and describes a very important aspect of strain in the performance of olivine cathode materials.
Abstract
Alkali ion intercalation compounds used as battery electrodes often exhibit first-order phase transitions during electrochemical cycling, accompanied by significant transformation strains. Despite ∼30 years of research into the behavior of such compounds, the relationship between transformation strain and electrode performance, especially the rate at which working ions (e.g., Li) can be intercalated and deintercalated, is still absent.
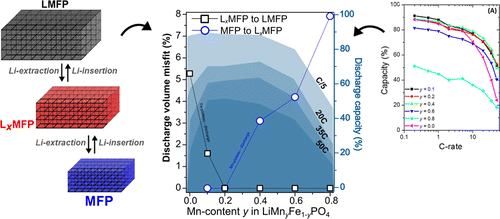 In this work, we use the LiMnyFe1–yPO4system for a systematic study, and measure using operando synchrotron radiation powder X-ray diffraction (SR-PXD) the dynamic strain behavior as a function of the Mn content (y) in powders of ∼50 nm average diameter. The dynamically produced strain deviates significantly from what is expected from the equilibrium phase diagrams and demonstrates metastability but nonetheless spans a wide range from 0 to 8 vol % with y. For the first time, we show that the discharge capacity at high C-rates (20–50C rate) varies in inverse proportion to the transformation strain, implying that engineering electrode materials for reduced strain can be used to maximize the power capability of batteries.
In this work, we use the LiMnyFe1–yPO4system for a systematic study, and measure using operando synchrotron radiation powder X-ray diffraction (SR-PXD) the dynamic strain behavior as a function of the Mn content (y) in powders of ∼50 nm average diameter. The dynamically produced strain deviates significantly from what is expected from the equilibrium phase diagrams and demonstrates metastability but nonetheless spans a wide range from 0 to 8 vol % with y. For the first time, we show that the discharge capacity at high C-rates (20–50C rate) varies in inverse proportion to the transformation strain, implying that engineering electrode materials for reduced strain can be used to maximize the power capability of batteries.
