Storage of renewable energy is essential in order to create a new sustainable energy economy e.g. directly as electricity in a Li-battery or indirectly as hydrogen in a solid state metal hydride.1 Batteries are rechargeable but have moderate life time and limited energy storage capacity. Metal borohydrides can store considerable amounts of energy as hydrogen in the solid state, but tend to exhibit poor thermodynamic and kinetic properties, which hamper their technological utilization. Indeed, this calls for continued intense research within the energy storage materials science.2
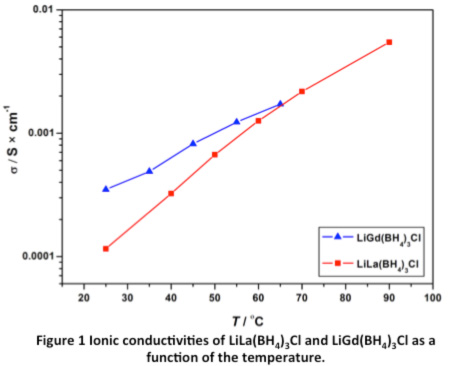
Recently, a system of novel rare earth metal borohydrides was discovered with composition LiM(BH4)3Cl (M = La, Gd).3-4 The new compounds all have the unique property of very high Li ion conductivity (σ ~ 1 × 10-4 S∙cm-1), see Figure 1. LiM(BH4)3Cl (M = La, Gd) both crystallize in the cubic crystal system with the space group I-43m and unit cell parameters a = 11.7955(1) and a = 11.5627(1), respectively. The structure contains isolated tetranuclear anionic clusters of [M4Cl4(BH4)12]4– with distorted cubane M4Cl4 cores, charge-balanced by Li+ cations. The Li+ ions are disordered on 2/3 of the 12d Wyckoff site, which agrees well with the very high lithium ion conductivity. Mechano-chemical synthesis from the corresponding rare earth metal chlorides (MCl3 M = La, Ce, Gd) and lithium borohydride (LiBH4) remains the only production option. Unfortunately, this method inevitably co-produces non-conducting LiCl. Preliminary results show that LiGd(BH4)3Cl have a high decomposition potential of 7 V and that a battery constructed using LiGd(BH4)3Cl as solid state electrolyte has electrochemical activity.
(1) Fichtner, M. J. Alloys Compd. 2011, 509, 529–534. (2) Rude, L. H.; Nielsen, T. K.; Ravnsbæk, D. B.; Bösenberg, U.; Ley, M. B.; Richter, B.; Arnbjerg, L. M.; Dornheim, M.; Filinchuk, Y.; Besenbacher, F.; Jensen, T. R. Phys. Status Solidi A 2011, 208, 1754–1773. (3) Ley, M. B.; Ravnsbæk, D. B.; Filinchuk, Y.; Lee, Y.-S.; Janot, R.; Cho, Y. W.; Skibsted, J.; Jensen, T. R. Chem. Mater. 2012, 24, 1654–1663. (4) Ley, M. B.; Boulineau, S.; Janot, R.; Filinchuk, Y.; Jensen, T. R. J. Phys. Chem. C 2012, 116, 21267–21276.